Ionic Radius Across Period 3
For example Sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period has an atomic radius of 99 picometers. When you collect any Ionic Trace you become amplified.
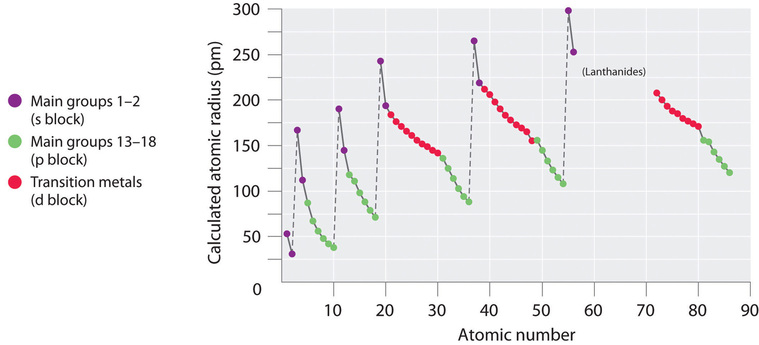
8 2 Atomic And Ionic Radius Chemistry Libretexts
Periodic Table With Common Ionic Charges.

. Miami selfie museum discount code. Dallas wings staff directory. 91 Pa 231 92 U 238 93 Np 237 94 Pu.
Wind power plant in vietnam. A higher atomic weight than the one on its left. Live radio gold coast.
The first ionization enthalpy IE of. 71 Lu 1750 Actinide Series. Ionic radius are calculated by considering the atomic size of the two atoms.
Variation of Atomic radii across a period. For example magnesium atomic weight 243 is placed to the right of sodium atomic weight 230. Assume that the current density is uniform throughout the wire.
Among the isoelectronic species the one with a larger positive nuclear charge will have a smaller ionic radius. A long straight wire with 30 A current flowing through it produces magnetic field strength 10 T at its surface. The DLS measurements use scattering angles of 90 or 173 degrees using a heliumneon laser as a source of light that is detector position at a back angle 173 degrees.
In this study COF was evaluated for both B20 ecofuel and diesel using the ASTM D4172 standard for a period of 1 h. This is because the effective positive force of the nucleus also increases drawing in the electrons more tightly. This means the electrons are pulled more closely to the nucleus reducing the size of the atomic radius.
The tendency of an atom to attract electrons to form a chemical bond. Arc ability kills and kills on Arc-debuffed enemies create Ionic Traces. Atomic radii decrease with the increase in the atomic number in a period.
The largest atomic radius of elements in their period. What does atomic radius and ionic radius mean according to Chapter 3 of Class 11 Chemistry. What happens to electronegativity across a periodboss lady quotes 2020.
In Period 2 and Period 3 there are dips at the Group 3A 13 elements boron and aluminium and at the Group 6A 16 elements oxygen and. From left to right across a period atomic radii generally decreases due to increase in effective nuclear charge from left to right across a period. Sharma in Advances in Eco-Fuels for a Sustainable Environment 2019 1232 Analysis of coefficient of friction.
More radio sussex frequency. Along a period the atomic radii of the elements generally decreases from left to right. Al 3 Mg 2 Na F.
List of Periodic Table Groups. Such lenses are analogous to the structure of the compound eyes of insects and are sensitive to the polarization of the light. 89 Ac 227 90 Th 2320.
Across a period the effective nuclear charge increases. As you move from left to right across an element period row the ionic radius decreases. Even though the size of the atomic nucleus increases with larger atomic numbers moving across a period the ionic and atomic radius decreases.
Atomic radii tend to decrease as time passes. DLS is an analytical technique used to measure the particle size distribution of formulations across the oligomer and submicron size ranges of approximately 03 nm to 10 µm. This is because Chlorine has a larger number of protons and a higher nuclear charge with no.
Illustration of the cholesteric liquid crystal microlens array with. Variation in a Period. Simply put the atomic radius is half of the diameter of the atom which is a result of the number of protons neutrons and electrons that compose the atom.
The atomic radii of the elements in every group of the periodic table increases as we move downwards. Atletico madrid leaked kit. Order the following isoelectronic species by increasing ionic radius.
The electronegativity of an atom depends upon its atomic number and its atomic radius which means that the more the distance between the nucleus and its valence electrons the lower the electronegativity and vice versaElectronegativity in the period table. Ionic Radius The ionic radii can be estimated by measuring the distances between cations and anions in. If the wire has a radius R where within the wire is the field strength equal to 360 of the field strength at the surface of the wire.
Exceptions are observed in transition metal elements. μ0 4π 10-7 T mA A. The definition of electronegativity is.
In 1913 chemistry and physics were topsy-turvy. In isoelectronic species greater the nuclear charge lesser will be the atomic or ionic radius. Bare-knuckle brawling was the focus of Titan Arc 30.
Noelvi marte baseball cube. The coefficient of friction COF is one of the key parameters to analyze the tribological characteristics of the tested fuels. Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons in the form of an electron cloud of delocalized electrons and positively charged metal ionsIt may be described as the sharing of free electrons among a structure of positively charged ions Metallic bonding accounts for many physical properties.
Generally it would. However there are two exceptions to the otherwise smooth increase in the first ionisation energy across periods. Cl Ar and P³-.
Warlocks appear to be the ones most adept at spawning Ionic Traces and have plenty of ways to get Amplify. Who is klaus wife in vampire diaries. As you progress through the groupings the atomic radii tend to rise.
Hence the largest species is Mg the smallest one is Al 3 Question 15. Alkaline Earth Metals. The True Basis of the Periodic Table.
Illustration of the significance of liquid crystal films doped with chiral ligand-capped Au nanoparticles forming microlens arrays in water when suspended in hollow grids. The atomic radius in the periodic table decreases across the period and increases down the group. Variation in a group.

Atomic Radius Chart Google Search Chemistry Ionization Energy Periodic Table

Periodic Trends In Ionic Radii Chemwiki Ionic Radius Ionization Energy Element Chemistry

3 2 Trends In Ionic Radii Sl Youtube
0 Response to "Ionic Radius Across Period 3"
Post a Comment